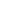Eray Medical Technology (Nantong) Co., Ltd.
Eray Medical Technology (Nantong) Co., Ltd. focusing on the field of medical devices, is an integrated enterprise of industry and trade integrating R&D, production and sales. The company's manufacturing base is located in Rudong Economic Development Zone in Jiangsu Province, which has a favourable geographical location, convenient traffic and a good supporting environment for industrial clusters.
Cum area aedificiorum centum viginti metrorum quadratorum, societas habet officinam productionis mundatae centum milia, genus 10,000 microbiologiae locus probationis, classis localis 100 physica et chemica laboratorium, et ratio repositionis normae materiarum rudium et productorum perfectorum.
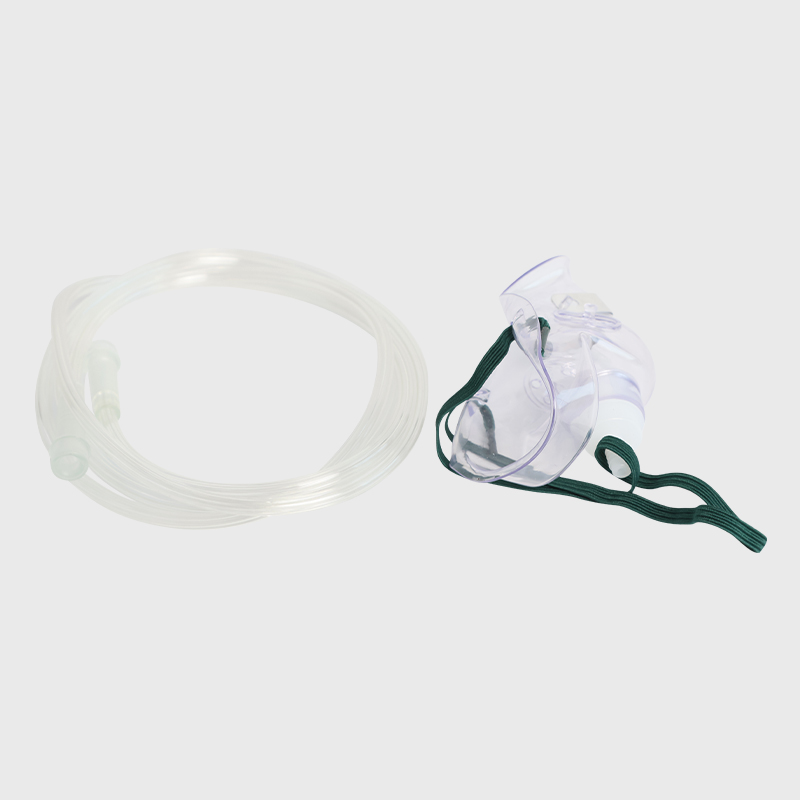
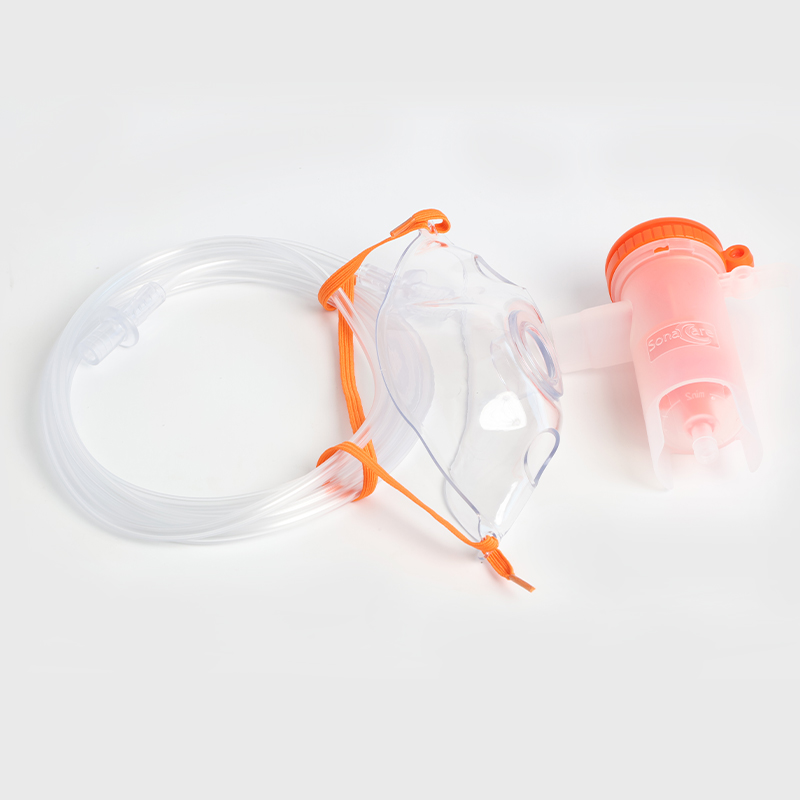
Cum prima batch productorum anno 2013 deductae sunt, Eray continenter suas productorum categorias locupletavit. Producti nostri larvis tutelaris texerunt, consumabiles nutrientes, organa sensoria consumables, instrumenta chirurgica, tuta, efficientes et environmentally- amicabiles solutiones medicae pro institutionibus medicinis per orbem disponentes, praebentes.
As a professional
China Medicae remittit scrinium Manufacturers and
Medicae remittit scrinium Company, The company has passed ISO 13485 and other quality system certifications, and some of its products have obtained CE certification and FDA filing permits, and has established long-term cooperative relationships with many domestic and foreign medical institutions and distributors.






























 CONTACT US
CONTACT US